What’s Ozone?
Ozone is one of the components found in the Earth’s atmosphere.
It belongs to the same family of gases as oxygen and carbon dioxide.
Ozone also exists in small amounts near the Earth’s surface, but about 90% of it is concentrated in the stratosphere.
This is what we call the ozone layer.
It plays an important role in blocking harmful ultraviolet rays from the sun.
In the stratosphere, ozone exists in a gaseous form.
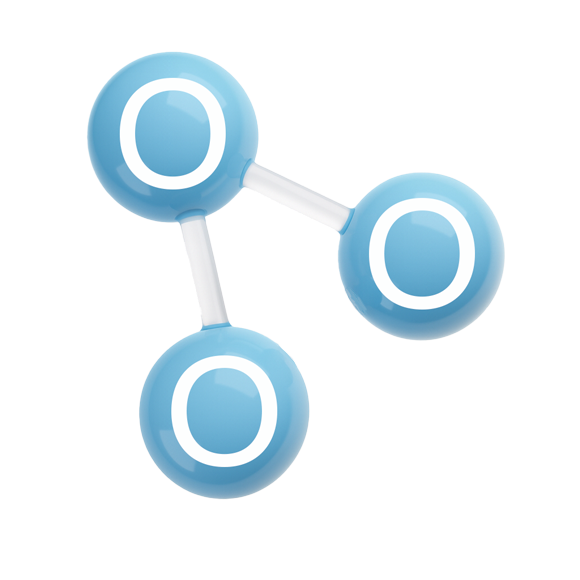
Ozone is made up of three oxygen atoms (O).
Its molecular formula is O₃, meaning it consists of three oxygen atoms.
For comparison, oxygen gas consists of two oxygen atoms (O₂), and water is made of two hydrogen atoms and one oxygen atom (H₂O).
Ozone is a very simple natural substance, made from the same basic elements as water and oxygen.
However, its characteristics can vary greatly depending on its state.
When Ozone is in its gaseous form
- It has a distinct, strong odor.
- At high concentrations, it can be harmful to humans.
- It is also heavier than oxygen.
When ozone is in its liquid form — ozonated water —
- It does not emit any harmful gases.
- It has powerful effects such as sterilization, bleaching, deodorization, and decomposition of harmful substances.
- It leaves no residue, as it naturally breaks down into oxygen and water.
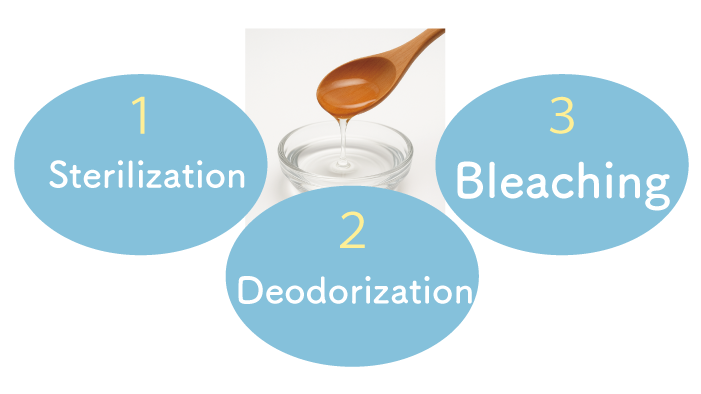
Characteristics of Ozone
Ozone has three main effects:
- Sterilization: It destroys the cell walls and membranes of bacteria, viruses, and molds through its strong oxidizing power.
- Deodorization: It breaks down odor-causing substances using oxidation.
- Bleaching: It whitens by decomposing pigments and organic substances through oxidation.
Ozone is widely used in practical applications.
Use in water and food:
Many water treatment plants, including those in Tokyo, use ozone to purify tap water.
Ozonated water is also used for washing convenience store salads, rice balls, and leafy vegetables.
Use in medical institutions:
Ozonated water is used as a cleansing solution in procedures such as eye surgeries.
It is safe enough to be used on delicate areas of the body.
In addition, ozone gas is utilized for disinfecting ambulances.
Use in coin laundries:
By using ozonated water during the rinsing process, bacteria attached to clothing can be eliminated and odors reduced.
This helps keep garments cleaner and prevents unpleasant smells.
Use in paper and towels:
By harnessing ozone’s bleaching power, pure white towels and bright white paper are produced.
What is Ozonation?
“Ozone reaction,” or ozonation, is a technique that uses the powerful oxidizing ability of ozone (O₃) to chemically modify organic substances or surfaces.
It is widely applied in fields such as environmental engineering, medicine, and materials science.
① Characteristics of Ozone Reactions
- [Strong Oxidizing Power]
Ozone has a high oxidation–reduction potential of 2.07 V, making it the second most powerful oxidizing agent after fluorine. - [Selective Reactivity]
It is effective for modifying olefins (C=C bonds) and aromatic rings, as well as for introducing various functional groups. - [Usable at Room Temperature and Pressure]
Ozone can be applied under normal conditions and is often used in combination with ultraviolet irradiation or plasma generators to act on surfaces. - [Few By-products]
Since ozone naturally reverts to oxygen after the reaction, it generates minimal by-products and imposes a low environmental burden.
② Applications
- [Environmental Purification]
Used in water treatment to decompose organic pollutants and to enhance the hydrophilicity of membranes. - [Food Packaging]
Improves preservation by increasing surface hydrophilicity and adding antibacterial properties to polymers. - [Pre-adhesion Treatment]
Applied to automotive parts, electronic components, and medical devices to improve adhesion performance before bonding.