The world’s leading academic journal on cosmetic dermatology「Journal of Cosmetic Dermatology」has accepted Mediplus Pharma’s article ” Ozonized glycerin (OG)-based cosmetic products lighten age spots on human facial skin“”
Mediplus Pharma, Inc. (CEO: Kenji Ito, Head Office: Shibuya-ku, Tokyo,JAPAN) has announced that “Human Clinical Study Results of Ozonized Glycerin” has been accepted by the Journal of Cosmetic Dermatology, an American academic journal on cosmetic dermatology and human clinical studies and published in May 2022.
Title: Ozonized glycerin (OG)-based cosmetic products lighten age spots on human facial skin
Background:
Few cosmetic ingredients are shown to be able to safely remove or lighten facial dark spots once they have formed.
OG has been reported to possess oxidation power and exhibit various biological activities such as antibacterial, antivi- ral, and wound healing promotion.
Purpose:
This study aimed to clarify the effects of OG on human skin, especially on age spots on the face.
Method:
OG formulations (80 and 800 ppm) were mixed with synthetic melanin in vitro for 4 weeks and then assessed for its ability to degrade the melanin. OG also investigated its effect on gene expression of keratinocyte differentiation markers in vitro to explore the cell maturation. In clinical study for the evaluation of effects of OG formulations on age spots on facial skin, 48 women were measured for the melanin content of them by a Mexameter at 4 and 8 weeks after daily twice application of OG formulations. Adverse events were monitored during the study.
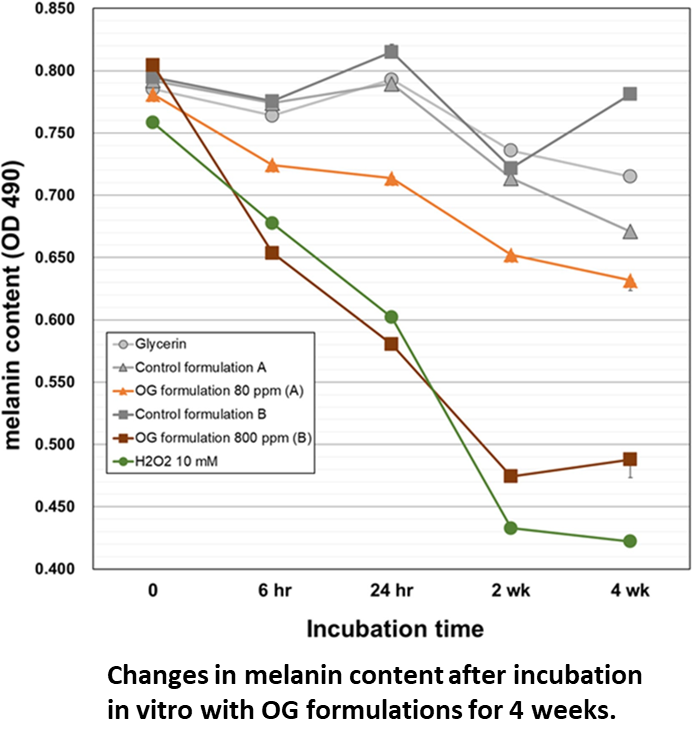
Results:
Both OG formulations showed direct melanin degradation in a time-dependent manner, with significant effects observed as early as 6 h. OG formulation at 800 ppm showed higher activity than OG formulation at 80 ppm, and the amount of melanin was decreased by about 40% on Day 14 of the mixing reaction.
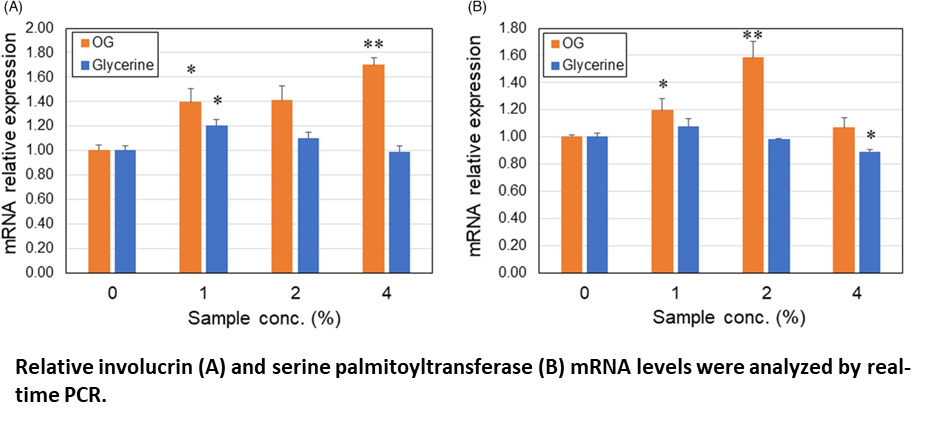
Differentiation marker studies using human keratinocytes showed that the gene expression of involucrin and serine palmitoyltransferase was upregulated by OG, which was almost equivalent con- centration to OG formulation 80 ppm, suggesting that OGs can enhance turnover of the skin epidermis.
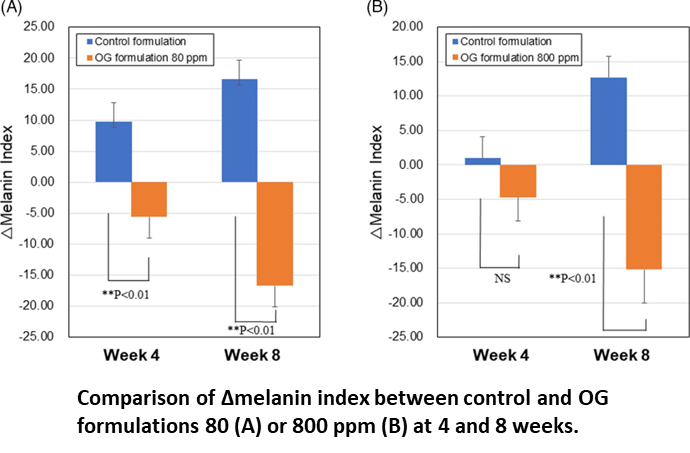
In clinical study, OG formulations 80 and 800 ppm showed larger decreases in melanin contents at 8 weeks compared with those at 4 weeks and their mean values of △melanin index were −16.7 and −15.2, respectively.Statistically sig- nificant differences were detected against respective controls. Number of subjects with a decrease in melanin index from baseline to 4 or 8 weeks increased in both OG formulations 80 and 800 ppm, especially prominent at 8 weeks. There were no ad- verse events related to treatments of OG 80 and 800 ppm during the study.
Conlusion:
The result indicated that applications of OG formulations are safe and effective in lightening age spots on the facial skin.
What is Journal of Cosmetic Dermatology
The Journal of Cosmetic Dermatology is the official journal of the International Academy of Cosmetic Dermatology (IACD) and the Canadian Association of Aesthetic Medicine (CAAM).
The Journal publishes high quality, peer-reviewed articles on all aspects of cosmetic dermatology with the aim to foster the highest standards of patient care in cosmetic dermatology. Published monthly, the Journal of Cosmetic Dermatology facilitates continuing professional development and provides a forum for the exchange of scientific research and innovative techniques.
*




