The Effect of Ozone on Collagen Type-1 and Inflammatory Cytokine Production in Human Gingival Fibroblasts
Yoshimasa Makita1, Yasuhiro Imamura2, Kazuya Masuno3, Isao Tamura4, Shin-ichi Fujiwara1, Gotaro Shiota5, Akihiko Shiba6 and Pao-Li Wang7*
1Department of Chemistry, Osaka Dental University, Osaka, Japan
2Department of Pharmacology, Matsumoto Dental University, Nagano, Japan
3Department of Innovation in Dental Education, Osaka Dental University, Osaka, Japan
4Department of Oral Anatomy, Osaka Dental University, Osaka, Japan
5VMC Co., Ltd., Tokyo, Japan (currently Mediplas Pharmaceuticals)
6Department of Prosthodontics, Faculty of Dentistry, Showa University, Tokyo, Japan
7Department of Bacteriology, Osaka Dental University, Osaka, Japan
Abstract
Ozone is currently being considered as a possible oral antiseptic agent because it is strongly antimicrobial and does not induce microbial resistance. In the article, we examined the effects of ozone exposure on the production of collagen type-1 and inflammatory cytokines in primary human gingival fibroblasts (HGFs) in vitro using enzyme-linked immunosorbent assays. The addition of 0.5 ppm ozone significantly enhanced collagen type-1 production by HGFs within 24 h. Secretion of the pro-inflammatory cytokines interleukin-6 (IL-6) and IL-8 by HGFs treated with lipopolysaccharide decreased when ozone was present in the medium. Together, these results suggest that clinical use of ozone would facilitate the positive balance between HGF-mediated periodontal tissue maintenance
Keywords:
Ozone; Collagen; Periodontal disease; Human gingival fibroblasts
Introduction
Ozone is currently being considered in dentistry as a possible alternative oral antiseptic agent. Its strong antimicrobial effect without the development of drug resistance has been previously noted in water purification and food preservation techniques[1-3]. In dentistry, ozone has been used either gaseous or aqueous forms for the elimination of caries pathogens, in the disinfection of root canals, and as a rinse for avulsed teeth [4-8]. However, ozone has an unpleasant smell and a short half-life of about 40 min [9]. Ozone also has low water solubility and thus, aqueous ozone formulations provide no long-term sterilization effect. On the other hand, ozone gel (VMC Co., Ltd., Tokyo, Japan), which consists of a glycerin solution containing ozone, has a long-term sterilization effect. The advantages of ozone gel include a 6-month-long sterilization effect, the lack of an unpleasant smell, and no development of bacterial strains manifesting ozone-resistance. In the course of our studies, we have reported the safety evaluation of ozone for the skin and eye, as well as its antimicrobial effects and hemostasis using ozone gel [10-12]
In addition, a number of reports have shown that ozone could cause improvements in periodontal diseases [13-15][13-15]。
However, the effects of ozone on the functions of the cells involved in periodontal disease have yet to be elucidated [16-19].[16-19]。
Periodontal diseases are caused by a number of cells and cytokine networks, and the contributing factors are complex; therefore, the collection of data from basic research studies using cultured cells is an important tool for understanding disease development and progression. Thus, in this study, we examined the effects of ozone on the production of inflammatory cytokines and type I collagen in human gingival fibroblasts (HGFs) in vitro, and attempted to elucidate the mechanism of action of ozone on periodontal disease.
Materials and Methods
Cell cultures
HGF cells were cultured in Dulbecco’s modified Eagle medium (DMEM, Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) with 10%.fetal bovine serum (FBS), 100 units/ml penicillin G, and 100 μg/ml streptomycin at 37ºC in a 5% CO2 and 95% air humidified incubator. HGFs used in this study were obtained from volunteers after appropriate informed consent was obtained. The Ethics Committee of Osaka Dental University approved the study (protocol 110778). HGFs isolated from adherent gingival tissue on the extracted teeth of patients with chronic periodontal were cultured on collagen-coated plates in medium.
Reagents
The following materials and antibodies were purchased: 100 ppm Ozone gel (VMC Co., Ltd.); lipopolysaccharide (LPS) from Porphyromonas gingivalis (P. gingivalis) (InvivoGen, San Diego, CA, USA); anti-Interleukin (IL)-6 and biotinylated anti-IL-6 antibodies (eBiosciences, San Diego, CA, USA); anti-IL-8 and biotinylated anti- IL-8 antibodies (R&D Systems, Minneapolis, MN, USA); biotinylated anti-collagen type I antibody (Rockland, Limerick, PA, USA); and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO, USA); BrdU (5-bromo-2′- deoxyuridine) Cell Proliferation Assay kit (Millipore, Billerica, MA, USA).
DNA synthesis and MTT assays
For analysis of DNA synthesis, HGFs (1 × 104/cm2) were cultured in DMEM containing 0.5% FBS (0.5% DMEM) for 24 h. The cells were then cultured with diluted ozone gel at 0.05, 0.5, and 5 ppm for 2 min, and the culture medium was removed. Then, the cells were washed with 0.5% DMEM and were cultured with 0.5% DMEM containing BrdU for 24 h. The level of DNA synthesis in the cells was determined by measuring BrdU-incorporation using the BrdU Cell Proliferation Assay kit. For MTT assay, cells were cultured with the ozone gel at the above concentrations in DMEM containing 10% FBS (10% DMEM) for 2 min, and the culture medium was removed. Then the cells were cultured with 10% DMEM for 24 h after washing with 10% DMEM. The subsequent procedures were performed as described elsewhere [20].[20]。
Enzyme-linked immunosorbent assays (ELISA)
To detect cytokine production, HGFs (1 × 104/cm2) were cultured with the ozone gel (0.5 ppm) for 2 min, and then the culture medium was removed. The cells were cultured with P. gingivalis LPS (100 ng/ ml) for 24 h after washing with 10% DMEM. Then, the culture media were collected, and the cytokine levels were measured using the anti- IL-6 (1 μg/ml) and biotinylated anti-IL-6 (0.6 μg/ml), or anti-IL-8 (2.5 μg/ml) and biotinylated anti-IL-8 (0.2 μg/ml) antibodies. For collagen production, HGFs (1 × 104/cm2) were cultured in DMEM containing 1% FBS (1% DMEM) with the ozone gel (0.5 ppm) for 2 min. The cells were then washed and cultured with 1% DMEM. Levels of collagen type I were measured using the biotinylated anti-collagen type I antibody (0.2 μg/ml). ELISAs was performed as described in the user manual of the CytoSet kits (Biosource International, Camarillo, CA, USA) [21][21]. The cells for collagen production were lysed with 0.5% TritonX-100, and the amounts of proteins from the cell lysates were measured using a BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA). Collagen production was normalized to the total protein content of the cell lysates.。
Statistical analysis
Quantitative data were statistically analyzed using either one-way analysis of variance (ANOVA) followed by a Tukey’s test or a Student’s t-test. Differences were considered to be significant at p<0.05.
Results
The effects of ozone on the cell viability of HGFs were examined using an MTT assay (Figure 1). Up to 0.5 ppm dosage, the ozone showed no marked cytotoxicity against HGF, but at 5 ppm, it caused a decrease in cell viability by approximately 20% in comparison with untreated controls. These findings suggested that ozone at this level might be cytotoxic against HGF. Therefore, the conditions of this study were narrowed down to a range within which the ozone did not exhibit cytotoxicity against HGF; and the following experiments were carried out using ozone at 0.5 ppm.
We next evaluated the effects of ozone on the type-I collagen production ability of HGFs, which is directly linked to the regenerative capacity of periodontal tissues (Figure 2). This level of ozone, which promoted cell proliferation in comparison with the controls during the evaluation of cell viability, also enhanced the production of type-I collagen by HGFs by approximately 1.6-fold.
The effects of ozone on the secretion of inflammatory cytokines by HGFs are shown in Figure 3. The production of IL-6 and IL-8 by HGFs was markedly promoted when the latter were placed under the stimulation of LPS from the periodontal pathogenic bacterium P. gingivalis, which was known to have a strong inflammation-inducing effect. In contrast, the production of interleukins was markedly suppressed when the stimulation was carried out in the presence of ozone.
Discussion
Periodontal diseases, which have been found to be associated with lifestyle-related diseases, are biological responses triggered by complex interactions between the host and periodontal pathogenic bacteria living in the oral cavity, as well as by mechanical stress. In general, the destruction of periodontal tissues and the progression of the damage are due to various cytokines released by cells in the periodontal tissues concentration of cytokines such as interleukins IL-6 and IL-8 are known to increase in inflammatory periodontal tissues [22].[22]
IL-6 has multiple functions; in addition to its regulatory function on the differentiation and proliferation of immune cells [23], [23]、it also aggravates periodontal diseases by inducing bone resorption [24].[24]。
IL-8 is believed to be involved in the destruction of periodontal tissues by inducing neutrophils [25,26].[25-26]
Furthermore, various cells, including macrophages and lymphocytes, are known to be intricately involved in the aggravation of periodontal diseases. Among these, HGFs are the most abundantly present cells in gingival connective tissues. HGFs assume the production of collagen, the construction of the structure of periodontal tissues, and the maintenance of homeostasis [27].[27]
When they are exposed to pathogenic bacteria or to their components such as LPS, they secrete inflammatory cytokines including IL-6 and IL-8 and thus induce the destruction of periodontal tissues [27,28].[27-28]In addition, the secretion of these cytokines has also been reported to be strongly involved in chronic inflammation [29].[29] Our in vitro study has confirmed that ozone enhanced the production of type-I collagen by HGFs, and inhibited the LPS-induced production of inflammatory cytokines (IL-6 and IL-8) therefrom (Figure 3). Furthermore, HGFs have previously been reported to express the genes for Toll-like receptors (TLR) 1-5 and 9, which are widely known as receptors for pathogens; in particular, TLR-2,3,4 and 5 have been reported to be specifically involved in the expression of IL-8 [30],[30]and TLR-2 is known to be involved in the expression of IL-6 [31].[31]Thus, these results support the finding of enhanced production of these cytokines following pathogen stimulation.
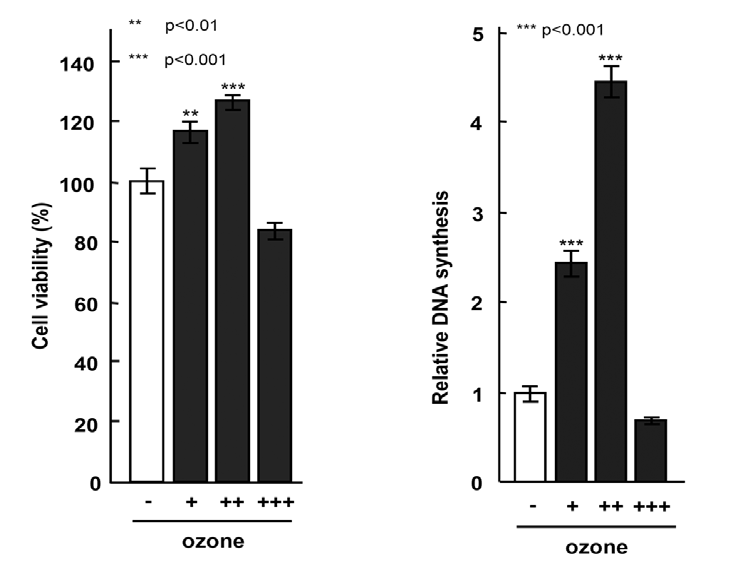
Human gingival fibroblasts were were exposed to media containing 0.05, 0.5, or 5 ppm ozone gel (designated as +, ++, and +++). All data were compared with those for cells treated with control medium without ozone gel. Data have been provided as mean ± SD (n=5). **p<0.01 and ***p<0.001, analyzed with ANOVA with a Dunnett’s test (vs. controls).
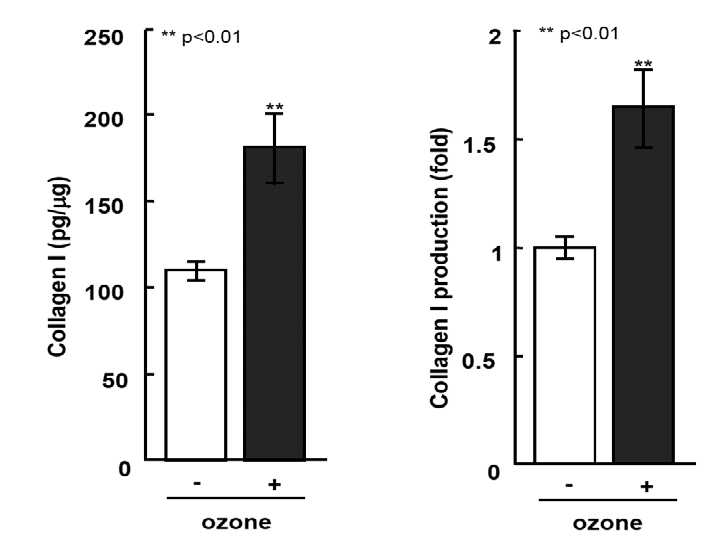
Human gingival fibroblasts were exposed to media containing 0.5 ppm ozone. Data have been provided as means ± SD (n=5). **p<0.01, analyzed with a Student’s t-test (vs. controls).
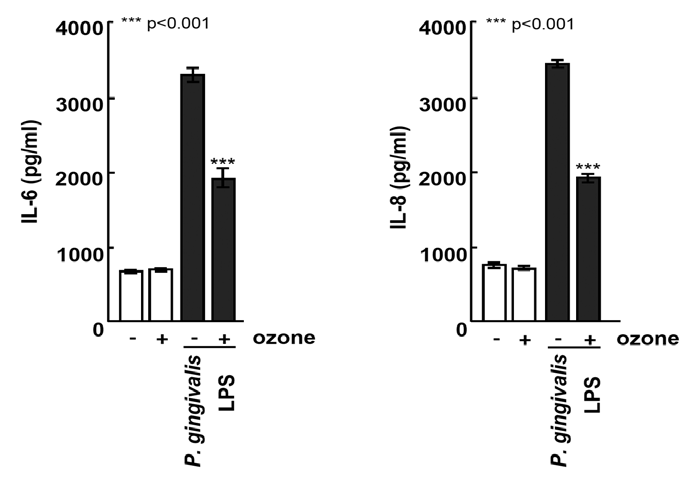
Human gingival fibroblasts were exposed to media containing 0.5 ppm ozone. The IL-6 and IL-8 concentrations in the supernatants were measured using ELISA. Data have been provided as mean ± SD (n=5). ***p<0.001, analyzed with ANOVA with a Tukey’s test. Statistical differences among ozone (+/-) exposures upon LPS administration are shown in the figure.
Conclusions
In conclusion, we demonstrated that ozone increased collagen type-1 production and hindered pro-inflammatory cytokine secretion from primary HGFs in vitro. In HGFs, cell growth and DNA synthesis were promoted by 0.5 ppm ozone, as was type-I collagen production. In contrast, this ozone dosage inhibited the production of IL-6 and IL-8 by HGFs that was induced by stimulation using P. gingivalis LPS. Together, these results suggest that clinical ozone use would facilitate the positive balance between HGF-mediated periodontal tissue maintenance and repair and the stimulation of inflammation and tissue degeneration following exposure to microbial pathogens.
References
- Paraskeva P, Graham NJ. (2002) Ozonation of municipal wastewater effluents. Water Environ Res 74: 569-581.
- Restaino L, Frampton EW, Hemphill JB, Palnikar P. (1995) Efficacy of ozonated water against various food-related microorganisms. Appl Environ Microbiol 61: 3471-3475.
- Unal R, Kim JG, Yousef AE. (2001) Inactivation of Escherichia coli O157: H7, Listeria monocytogenes and Lactobacillus leichmannii by combinations of ozone and pulsed electric field. J Food Prot 64: 777-782.
- Baysan A, Lynch E. (2004) Effect of ozone on the oral microbiota and clinical severity of primary root caries. Am J Dent 17: 56-60.
- Ebensberger U, Pohl Y, Filippi A. (2002) PCNA expression of cementoblasts and fibroblasts on the root surface after extraoral rinsing for decontamination. Dent Traumatol 18: 262-266.
- Huth KC, Paschos E, Brand K, Hickel R. (2005) Effect of ozone on non-cavitated fissure carious lesions in permanent molars. A controlled prospective clinical study. Am J Dent 18: 223-228.
- Kumar M, Haldia A, Gupta R, Meena D. (2015) Ozone therapy in dentistry- a review. J Sci Tech 1: 181-185.
- Nogales CG, Ferrari PH, Kantorovich EO, Lage-Marques J. (2008) Ozone therapy in medicine and dentistry. J Contemp Dent Pract 9: 75-84.
- Bocci V. (2002) Oxygen-ozone therapy: a critical evaluation. Springer Science & Business Media, Dordrecht.
- Wang PL, Shiota G, Shiba A. (2011) Safety evaluation of ozone gel for skin and eye on animal experiments. J Hard Tissue Biol 20: 313-318.
- Fukui T, Masuno K, Makita Y, Fujiwara SI, Shiota G, et al. (2014) Antimicrobial effects of ozone gel against periodontal bacteria. J Hard Tissue Biol 23: 445-448.
- Sakai D, Makita Y, Masuno K, Fujiwara SI, Okazaki J, et al. (2014) Local hemostatic effect of aqueous ozone in cutting wound surface. J Hard Tissue Biol 23: 245-248.
- Srikanth A, Sathish M, Sri Harsha AV. (2013) Application of ozone in the treatment of periodontitis. J Pharm Bioallied Sci 5: S89-S94.
- Saini R. (2011) Ozone therapy in dentistry: A strategic review. J Nat Sci Biol Med 2: 151-153.
- Arpita R, Swetha JL, Babu MR, Sudhir R. (2014) Recent trends in non-surgical periodontal care for the general dentist-a review. Bangl J Dent Res Edu 4: 78-82.
- Wang G, Umstead TM, Phelps DS, Al-Mondhiry H, Floros J. (2002) The effect of ozone exposure on the ability of human surfactant protein a variants to stimulate cytokine production. Environ Health Perspect 110: 79-84.
- Li Z, Tighe RM, Feng F, Ledford JG, Hollingsworth JW. (2013) Genes of innate immunity and the biological response to inhaled ozone. J Biochem Mol Toxicol 27: 3-16.
- Huang W, Wang G, Phelps DS, Al-Mondhiry H, Floros J. (2004) Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: effect of ozone-induced SP-A oxidation. Am J Physiol Lung Cell Mol Physiol 286: 546-553.
- Mikerov AN, Umstead TM, Gan X, Huang W, Guo X, et al. (2008) Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol 294: 121-130.
- Imamura Y, Fujigaki Y, Oomori Y, Usui S, Wang PL. (2009) Cooperation of salivary protein histatin 3 with heat shock cognate protein 70 relative to the G1/S transition in human gingival fibroblasts. J Biol Chem 284: 14316-14325.
- Imamura Y, Wang PL. (2014) Salivary histatin 3 inhibits heat shock cognate protein 70-mediated inflammatory cytokine production through toll-like receptors in human gingival fibroblasts. J Inflamm (Lond) 11: 4.
- Noh MK, Jung M, Kim SH, Lee SR, Park KH, et al. (2013) Assessment of IL‑6, IL‑8 and TNF‑α levels in the gingival tissue of patients with periodontitis. Exp Ther Med 6: 847-851.
- Hirano T, Akira S, Taga T, Kishimoto T. (1990) Biological and clinical aspects of interleukin 6. Immunol Today 11: 443-449.
- Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, et al. (1990) Interleukin-6 is produced by osteoblasts and induces bone resorption. J Immunol 145: 3297-3303.
- Okada H, Murakami S. (1998) Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med 9: 248-266.
- Wentworth P Jr, McDunn JE, Wentworth AD, Takeuchi C, Nieva J, et al. (2002) Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science 298: 2195-2199.
- Ara T, Fujinami Y, Urano H, Hirai K, Hatori T, et al. (2012) Protein kinase A enhances lipopolysaccharide-induced interleukin-6, interleukin-8 and PGE2 production by human gingival fibroblasts. J Periodontal Res 44: 21-27.
- Wang PL, Ohura K, Fujii T, Oido-Mori M, Kowashi Y, et al. (2003) DNA microarray analysis of human gingival fibroblasts from healthy and inflammatory gingival tissues. Biochem Biophys Res Commun 305: 970-973.
- Ara T, Kurata K, Hirai K, Uchihashi T, Imamura Y, et al. (2009) Human gingival fibroblasts are critical in sustaining inflammation in periodontitis. J Periodontal Res 44: 21-27.
- Mahanonda R, Sa-Ard-Iam N, Montreekachon P, Pimkhaokham A, Yongavinichit K, et al. (2007) IL-8 and IDO expression by human gingival fibroblasts via TLRs. J Immunol 178: 1151-1157.
- Souza PP, Palmqvist P, Lundgren I, Lie A, Costa-Neto CM, et al. (2010) Stimulation of IL-6 cytokines in fibroblasts by toll-like receptors 2. J Dent Res 89: 802-807.