The Effect of Ozone Gel on Bone Matrix Production by Human Osteosarcoma Cell Line Saos-2
Pao-Li Wang1)*, Yoichi Tachi2)*, Kazuya Masuno1), Nobutaka Okusa3) and Yasuhiro Imamura4)
1) Department of Innovation in Dental Education, Osaka Dental University, Osaka, Japan
2) Laboratory of Nutritional Physiology, Tokyo Kasei University, Tokyo, Japan
3) Department of Forensic Dentistry, Osaka Dental University, Osaka, Japan
4) Department of Dental Pharmacology, Matsumoto Dental University, Shiojiri, Japan
(Accepted for publication, April 20, 2018)
Abstract
Ozone is currently being considered as a potential oral antiseptic agent because it is highly antimicrobial and does not induce microbial resistance. In this study, we demonstrated that an optimal dosage of ozone gel enhanced the proliferation, type 1 collagen production, and alkaline phosphatase (ALP) secretion of Saos-2 cells in vitro. Proliferation of Saos-2 cells was assessed by MTT and DNA synthesis assays. Type 1 collagen production and ALP secretion were evaluated using enzymelinked immunosorbent assay (ELISA) and ALP assays. The cells were treated with/without 0.05, 0.5, 5 ppm ozone gel for 24 h. Ozone gel (0.5 ppm) signifi cantly induced the proliferation of Saos-2 cells. At this concentration, ozone gel enhanced type 1 collagen production and ALP secretion. The results indicated that ozone gel controls the cellular metabolism of osteoblasts, resulting in the secretion of early bone-related biomarkers.
Key words
Ozone gel, ALP, Collagen, Osteoblast, Saos-2 cells
Introduction
Ozone is currently being considered in dentistry as a potential alternative oral antiseptic agent. Its strong antimicrobial eff ect without the development of drug resistance has been previously noted in water purification and food preservation techniques1-3). In dentistry, ozone has been used either in gaseous or aqueous forms for the elimination of pathogens causing caries, in the disinfection of root canals, and as a rinse for avulsed teeth4-8). However, ozone has an unpleasant smell and a short half-life of approximately 40 min9). Ozone also has low water solubility, and therefore, aqueous ozone formulations provide no longterm sterilization effect. On the contrary, ozone gel, which consists of a glycerin solution containing ozone, has a long-term sterilization eff ect. The advantages of ozone gel include a 6-month-long sterilization eff ect, the lack of an unpleasant smell, and no development of bacterial strains manifesting ozone-resistance. Previously, we reported the safety evaluation of ozone for the skin and eye, as well as its antimicrobial eff ects and role in hemostasis using ozone gel10-12). In addition, a number of reports have shown that ozone can ameliorate periodontal diseases13-15). However, the effects of ozone on the functions of cells involved in periodontal disease are yet to be elucidated16-19). Recently, we reported the effects of ozone gel on the production of inflammatory cytokines and type I collagen in human gingival fi broblasts (HGFs) in vitro, and attempted to elucidate the mechanism of action of ozone on periodontal disease20). In this study, we examined effects of ozone gel on type 1 collagen production and alkaline phosphatase (ALP) secretion in the human osteosarcoma cell line Saos-2.
Materials and Methods
Cell cultures
Saos-2 cells (RIKEN BRC Cell Bank, Tokyo, Japan) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Nissui pharmaceutical Co. Ltd., Tokyo, Japan) with 10% fetal bovine serum (FBS), 100 units/ml penicillin G, and 100 μg/ml streptomycin at 37°C in a 5% CO2 and 95% air humidifi ed incubator.
DNA synthesis and MTT assays
For DNA synthesis, Saos-2 cells (1×104 ) were cultured in DMEM containing 0.5% FBS (0.5% DMEM) for 24 h. The cells were cultured with the ozone gel (VMC Co. Ltd. Tokyo, Japan) or at 0.05, 0.5, and 5 ppm for 2 min, and the culture medium was removed. Then, the cells were washed with 0.5% DMEM and were cultured with 0.5% DMEM containing bromodeoxy uridine (BrdU) for 24 h. The level of DNA synthesis was determined by measuring BrdU-incorporation using the BrdU cell proliferation assay kit (Millipore Tokyo, Japan).
For MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, Sigma- Aldrich) assay, cells were cultured with the ozone gel at 0.05, 0.5, and 5 ppm in DMEM containing 10% FBS (10% DMEM) for 2 min, and the culture medium was removed. After washing with 10% DMEM, the cells were cultured with 10% DMEM for 24 h. The subsequent procedures were performed as described elsewhere21).
Enzyme-linked immunosorbent assays (ELISA)
For collagen production, Saos-2 cells (1×104 ) were cultured in DMEM containing 1% FBS (1% DMEM) with the ozone gel (0.5 ppm) for 2 min. The cells were washed and cultured with 1% DMEM. Levels of type I collagen in the media were measured using the biotinylated antitype I collagen antibody (0.2 μg/ml, Rockland). ELISA was performed as described in the user manual of CytoSet kits (Biosource, Tokyo, Japan)22). The cells for collagen production were lysed with 0.5% TritonX-100, and the protein concentration of the cell lysates was measured using a bicinchoninic acid (BCA) protein assay kit (Pierce, Tokyo, Japan). Collagen production was normalized to the protein content of the cell lysates.
ALP activity
Saos-2 cells (1×104 ) were cultured with the ozone gel (0.5 ppm) for 2 min, and the culture medium was removed. The cells were washed with 0.5% DMEM and cultured with 10% DMEM for 24 h. The cells were lysed with 0.05% TritonX-100 and ALP activity of the lysates was measured using a LabAssay ALP kit (Wako Pure Chemicals Industries, Ltd., Osaka, Japan). The protein concentrations of the cell lysates were also measured using a BCA protein assay kit. ALP activity was normalized to the protein content of the cell lysates.
Statistical analysis
Quantitative data were statistically analyzed using either one-way analysis of variance (ANOVA) followed by Tukey’s test (DNA synthesis and MTT assays) or Student’s t-test (measurement of ALP activity and ELISA for collagen production) using the StatMate software (ATMS). Diff erences were considered to be signifi cant at p < 0.05.
J Hard Tissue Biology Vol. 27(3):195-198, 2018 27(3):195-198, 2018
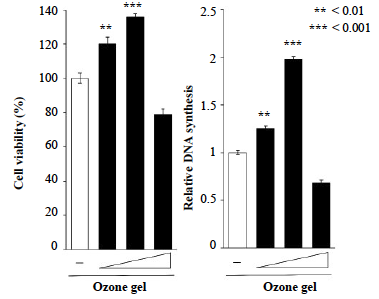
(a) MTT assay. (b) DNA synthesis assay. Saos-2 seeded at a volume of 3.1 × 104/ cm2 were exposed to media, which contain 0.05, 0.5, or 5 ppm ozone gel (designated as +, ++, and +++) for 24 h. All data were compared with those for cells treated with control medium without ozone gel. Data have been provided as mean ± S.D. (n = 3). **P < 0.01 and ***P < 0.001, analyzed with ANOVA with a Dunnett’s test (vs. none).
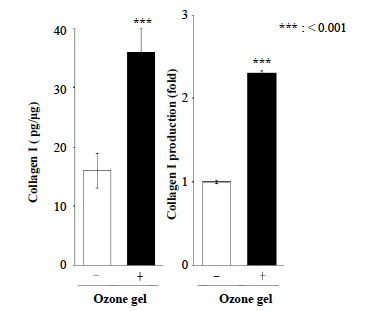
Saos-2 cells were seeded at a volume of 3.1 × 104/cm2 and were exposed to media containing 0.5 ppm Ozone gel for 24 h. Data have been provided as mean ± standard deviation (n = 3). ***P < 0.001, analyzed with Student’s t-test (vs. none).
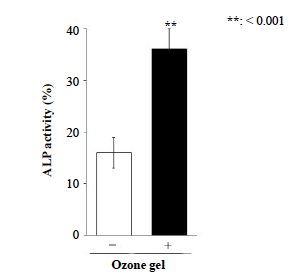
Saos-2 was seeded at 3.1 × 104/cm2 condition, and was exposed to media containing 0.5 ppm ozone gel for 24 h. Data have been provided as mean ± standard deviation (n = 3). **P < 0.01, analyzed with Student’s t-test (vs. none).
Results
Eff ect of ozone gel on proliferation of Saos-2 cells
After 24 h of incubation, the ozone gel treatment increased Saos-2 proliferation in a dose-dependent manner (Fig. 1). Ozone gel at 0.5 ppm signifi cantly induced cell growth compared to the other concentrations, whereas no obvious change was observed with 0.5 ppm ozone gel treatment. Similar to the results of the MTT assay, 0.5 ppm ozone gel eff ectively elevated DNA synthesis in Saos-2 cells, indicating the optimal concentration of ozone gel required for facilitating the proliferation of Saos-2 cells.
Eff ect of ozone gel on type 1 collagen production and ALP secretion by Saos-2 cells
Ozone gel at 0.5 ppm significantly induced cell growth. This, considering the results of the proliferation assays, we further evaluated the effect of ozone gel on collagen 1 production and ALP secretion from Saos-2 cells using 0.5 ppm ozone gel. Addition of ozone gel in the media eff ectively increased type 1 collagen production (Fig. 2) and ALP secretion (Fig. 3) from Saos-2 cells compared to that without ozone gel treatment.
Discussion
The eff ect of ozone gel on bone matrix production by osteoblasts is unclear. The present study showed that while high concentrations of the ozone gel decreased the cell viability index, low concentrations of the gel enhanced cell proliferation. Furthermore, 0.5 ppm ozone gel promoted the secretion of type 1 collagen and ALP, which are highly related to bone formation.
Previously, Oda et al. reported that 10 ppm ozone gel suppresses the proliferation of human gingival fibroblasts23). The present study showed that while cell proliferation was signifi cantly suppressed at high concentrations (5 ppm) of ozone gel, 0.5 ppm ozone gel promoted cell proliferation. In a study on non-gel forms of ozone, Tsujiue et al. reported that ozone water dissolved in phosphate-buff ered saline (PBS) induced cell damage at 3 ppm as visualized by hematoxylin and eosin staining24). A study using alveolar macrophages reported that 0.5 ppm ozone gas causes cellular damage to the monocyte strain THP-125). Ozone gel has the advantage of providing sustained release of ozone, which is contained within glycerin20). On the basis of these observations, we conclude that the ozone gel exhibits pharmacological effects without cytotoxicity at the available low concentrations and by further lowering the effective concentration via its sustained-release eff ect; however, it shows Holmins eff ect, i.e., cytotoxicity above a certain concentration.
Type 1 collagen and ALP are widely known as markers secreted in the early phases of bone formation20). In this study, these initial-phase bone matrix markers were secreted at an optimum concentration of the ozone gel. Currently, moderate levels of active oxygen species are thought to act as an intracellular signaling substance. Reactive oxygen species control intracellular signal transduction by chemically modifying proteins, nucleic acids, and lipids. Among these, participation of stress adaptation response by the Keep 1/Nrf 2 pathway is being actively researched. However, Nrf2 is known to negatively regulate the differentiation of osteoblasts. Therefore, it is highly likely that type 1 collagen secretion and increased expression of ALP observed in this study occurred via routes that did not involve Nrf2. Unfortunately, this study did not investigate the mechanism underlying the upregulation of these early bone matrix markers. Previous reports have identified that oxygen ozone therapy promotes the production of growth factors such as VEGF27). In addition, ozone gas is known to promote the production of FGF and other related cytokines28). VEGF enhances ALP expression in the mouse osteoblast cell line MC3T3-129). Thus, it is possible that bone matrix secretion may have been indirectly promoted via the secretion of these growth factors.
Our study demonstrates that 0.5 ppm ozone gel increases the secretion of osteoblast matrix without showing any signs of cell damage. Our previous reports have elucidated that the same concentration of ozone gel promotes secretion of collagen matrix and suppresses LPS-induced IL-6 and IL-8 expression without exhibiting cytotoxicity to human gingival fi broblasts20). Although it is diffi cult to directly link the results of in vitro and in vivo experiments, our results suggest that ozone gas, ozone water, ozone-olive oil30), and ozone gel may be used as therapeutic agents for periodontal disease. However, such applications will require detailed evaluation, such as assessment of biological safety using animal models and functional studies on its long-term eff ects in terms of cellular damage.
References
1. Paraskeva P and Graham NJ. Ozonation of municipal wastewater effl uents. Water Environ. Res 74: 569-581, 2002
2. Restaino L, Frampton EW, Hemphill JB and Palnikar P. Effi cacy of ozonated water against various food-related microorganisms. Appl Environ Microbiol 61: 3471-3475, 1995
3. Unal R, Kim JG and Yousef AE. Inactivation of Escherichia coli O157: H7, Listeria monocytogenes, and Lactobacillus leichmannii by combinations of ozone and pulsed electric fi eld. J Food Prot 64: 777-782, 2001
4. Baysan A and Lynch E. Eff ect of ozone on the oral microbiota and clinical severity of primary root caries. Am Dent 17: 56-60, 2004
5. Ebensberger U, Pohl Y and Filippi A. PCNA expression of cementoblasts and fibroblasts on the root surface after extraoral rinsing for decontamination. Dent Traumatol 18: 262-266, 2002
6. Huth KC, Paschos E, Brand K and Hickel R. Eff ect of ozone on noncavitated fissure carious lesions in permanent molars. A controlled prospective clinical study. Am J Dent 18: 223-228, 2005
7. Kumar M, Haldia A, Gupta R and Meena D. Ozone therapy in dentistry- a review. J Sci Tech 1: 181-185, 2015
8. Nogales CG, Ferrari PH, Kantorovich EO and Lage-Marques J. Ozone therapy in medicine and dentistry. J Contemp Dent Pract 9:75-84, 2008
9. Bocci V. Mechanism of action on ozone. In: Oxygen-ozone therapy.A critical evaluation, ed by Bocci V, Maruzen Publishing, Inc.,Tokyo, 2012, pp 17-25. (in Japanese)
10. Wang PL, Shiota G and Shiba A. Safety evaluation of ozone gel for skin and eye on animal experiments. J Hard Tissue Biol 20: 313-318, 2011
11. Fukui T, Masuno K, Makita Y, Fujiwara S and Shiota G.Antimicrobial effects of ozone gel against periodontal bacteria. J Hard Tissue Biol 23: 445-448, 2014
12. Sakai D, Makita Y, Masuno K, Fujiwara S and Okazaki J. Local hemostatic eff ect of aqueous ozone in cutting wound surface. J
Hard Tissue Biol 23: 245-248, 2014
13. Srikanth A, Sathish M and Sri Harsha AV. Application of ozone in the treatment of periodontal disease. J Pharm Bioallied Sci 5: S89-94,2013
14. Saini R. Ozone therapy in dentistry: A strategic review. J Nat Sci Biol Med 2: 151-153, 2011
15. Arpita R, Swetha JL, Babu MR and Sudhir R. Recent trends in nonsurgical periodontal care for the general dentist-a review. Bangl J
Dent Res Edu 4: 78-82, 2014
16. Wang G, Umstead TM, Phelps DS, Al-Mondhiry H and Floros J. The eff ect of ozone exposure on the ability of human surfactant protein a variants to stimulate cytokine production. Environ Health Perspect 110: 79-84, 2002
17. Li Z, Tighe RM, Feng F, Ledford JG and Hollingsworth JW. Genes of innate immunity and the biological response to inhaled ozone. J
Biochem Mol Toxicol 27: 3-16, 2013
18. Huang W, Wang G, Phelps DS, Al-Mondhiry H and Floros J. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: eff ect of ozone-induced SP-A oxidation. Am J Physiol-Lung Cell Mol Physiol 286: 546-553, 2004
19. Mikerov AN, Umstead TM, Gan X, Huang W, Guo X, Wang G,Phelps DS and Floros J. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol-Lung Cell Mol Physiol 294: 121-130, 2008
20. Makita Y, Imamura Y, Masuno K, Fujiwara S, Shiota G, Shiba A and Wang PL. The Eff ect of Ozone on Collagen Type-1 and Infl amatory Cytokine Production in Human Gingival Fibroblasts. Dentistry 5:339.doi:10.4172/2161-1122. 1000339, 2015
21. Imamura Y, Fujigaki Y, Oomori Y, Usui S and Wang PL. Cooperation of salivary protein histatin 3 with heat shock cognate protein 70 relative to the G1/S transition in human gingival fi broblasts. J Biol Chem 284: 14316-14325, 2009
22. Imamura Y and Wang PL. Salivary histatin 3 inhibits heat shock cognate protein 70-mediated inflammatory cytokine production through toll-like receptors in human gingival fi broblasts. J Infl amm (Lond) 11: 4, 2014
23. Oda H, Maruyama, K, Tsubokawa M, Sioda G, Kamoi H, Nagahiro K and Sato S. Eff ect of ozone gel on oral pathogens and human gingiva and periodontal ligament fi broblasts. Jpn J Conserv Den 57: 369-376, 2014
24. Tsujigami H. Sttudy of cytotoxic and bactericidal eff ects of ozonized water against periodontium-derived cells and periodontopathic bacteria. J Jpn Assoc Periodontol 44: 46-54, 2002
25. Klestadt D1, Laval-Gilly P and Falla J. Ozone-mediated cytotoxicity after short-term exposure and its relation to the production of cellular metabolites (NO, H2O2). Cell Biol Toxicol 18: 259-269, 2002
26. Miron RJ and Zhang YF. Osteoinduction: a review of old concepts with new standards. J Dent Res 91: 736-744, 2012
27. Zhang J, Guan M, Xie C, Luo X, Zhang Q and Xue Y. Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxid Med Cell Longev. 273475. doi: 10.1155/2014/273475, 2014
28. Rei L, martínez-sánchez G, perez-davison G and Sirito M. Role of ozone/oxygen in fi broblast growth factor activation. Discovering the Facts Int J Ozone Therapy 9: 55-58, 2010
29. Tan YY, Yang YQ, Chai L, Wong RW and Rabie AB. Effects of vascular endothelial growth factor (VEGF) on MC3T3-E1. Orthod Craniofac Res 13: 223-228, 2010
30. Srikanth A1, Sathish M and Sri Harsha AV. Application of ozone in the treatment of periodontal disease.J Phar Bioallied Sci 5: 89-94,2013


